Raleigh, NC, September 23, 2020 – QuadVantage Technologies, Inc. today announced that it has completed the development phase for its Quadriceps ACL Reconstruction System, a patented and clinically tested instrument set and procedure designed to transform ACL reconstruction outcomes by bringing the use of the Quadriceps tendon graft and corresponding superior patient benefits into the mainstream. The Company expects to make the specialized instrumentation and Quadriceps harvesting techniques commercially available during H1 2021, once it completes the final stage of biocompatibility, sterilization and packaging testing required by the FDA.
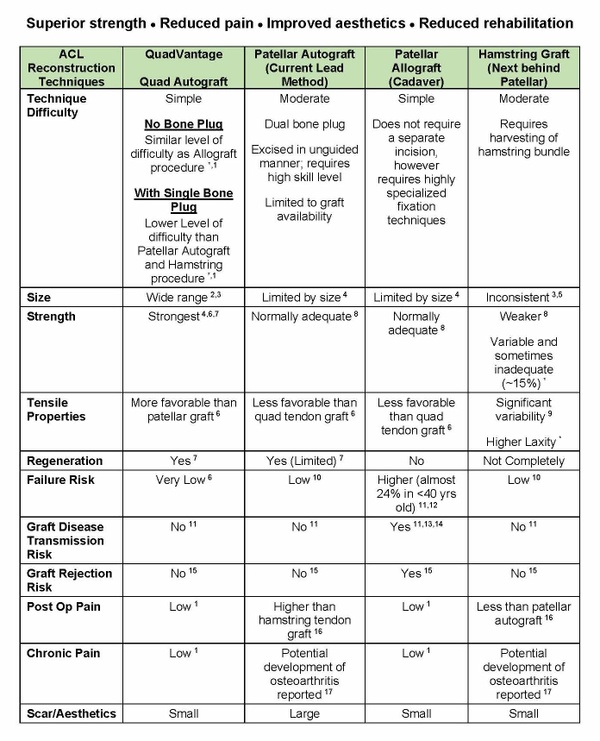
The QuadVantage System, based on results obtained to date, has been shown to deliver better results than current ACL reconstruction techniques that utilize Patellar, Hamstring or Allograft (cadaver) tendon grafts as replacements for the injured tendon. These results include:
- stronger grafts (lower risk of graft failure),
- reduced pain during recovery,
- less use of narcotics to relieve pain,
- reduced incidence of chronic pain,
- reduced rehabilitation, and
- lower risk of disease transmission (vs Allograft/cadaver grafts.)
“For more than 40 years published studies have shown that the Quadriceps tendon approach to ACL reconstruction can provide significant advantages when compared to Hamstring Patellar or Allograft (cadaver) ACL transplants, however the surgical complexity associated with harvesting and securing the Quadriceps tendon has prevented it from becoming a widely performed procedure,” said Dr. Paul Burroughs, III, M.D., QuadVantage founder and chairman of the board. “By simplifying the Quadriceps Autograft shaping and extraction process, the QuadVantage System delivers superior patient outcomes without added complexity or significantly increased cost.”
“The system that QuadVantage has developed is set to establish a new gold standard for ACL reconstruction by addressing key issues prevalent with existing methods,” said Tom Gutierrez, QuadVantage chief executive officer. “QuadVantage has been working for over 5 years to develop and clinically evaluate its simpler, more repeatable, and commercially viable approach. Patient and physician response have been very encouraging as we move to make our innovative system commercially available.”
A full comparative analysis of the advantages of the Quadriceps tendon versus other Autograft or Allograft (cadaver) alternatives currently used for ACL reconstruction has been posted on the Company website.
Safe Harbor Statements
Results noted are based on third party studies as well as QuadVantage’s experience over the course of its clinical evaluation. Please see references included under the Clinical Results tab on the Company’s website at https://quadvantage.com/clinical-results/.
All statements included in this press release other than statements of historical fact are forward-looking statements. Forward-looking statements are only predictions and are subject to many risks, uncertainties and other factors that may affect our businesses and operations and could cause actual results to differ materially from those predicted.
About QuadVantage Technologies, Inc.
QuadVantage is located in Raleigh, NC and was founded by Dr. Paul Burroughs, III., a board-certified orthopedic surgeon. The Company’s Quadriceps ACL Reconstruction System is protected by a series of granted and pending patents that cover its cutting edge QuadVantage instrument set as well as the procedural elements of its minimally invasive Quadriceps harvest techniques.